Hydrogen is a versatile energy carrier that can be used to power nearly every end-use energy need. The fuel cell — an energy conversion device that can efficiently capture and use the power of hydrogen — is the key to making it happen.
Fuel Cells are a cutting -edge technology for revolutionizing the clean energy. Fuel Cells are advanced energy conversion devices that generate electricity through chemical reaction between a fuel source (usually hydrogen) and an oxidizing agent (typically oxygen from the air). This process produces electricity, with water & heat as the only byproducts.
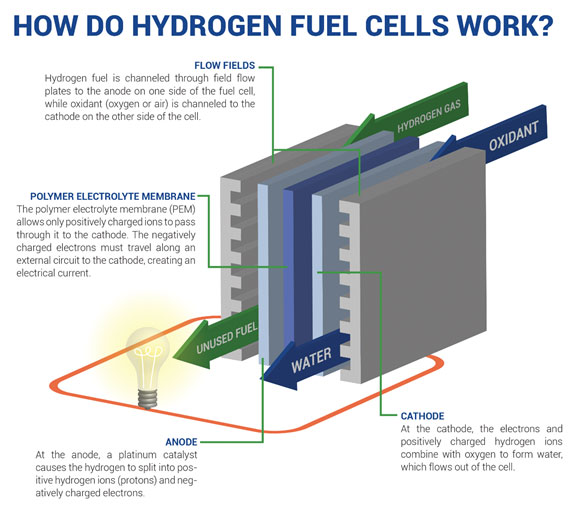
A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. In a hydrogen fuel cell, a catalyst at the anode separates hydrogen molecules into protons and electrons, which take different paths to the cathode. The electrons go through an external circuit, creating a flow of electricity.

Fuel cells represent a ground-breaking leap in electrochemical power generation. They harness the extraordinary potential of combining hydrogen with oxygen from the air to yield electricity, while leaving behind just two environmentally benign by-products: water and heat.
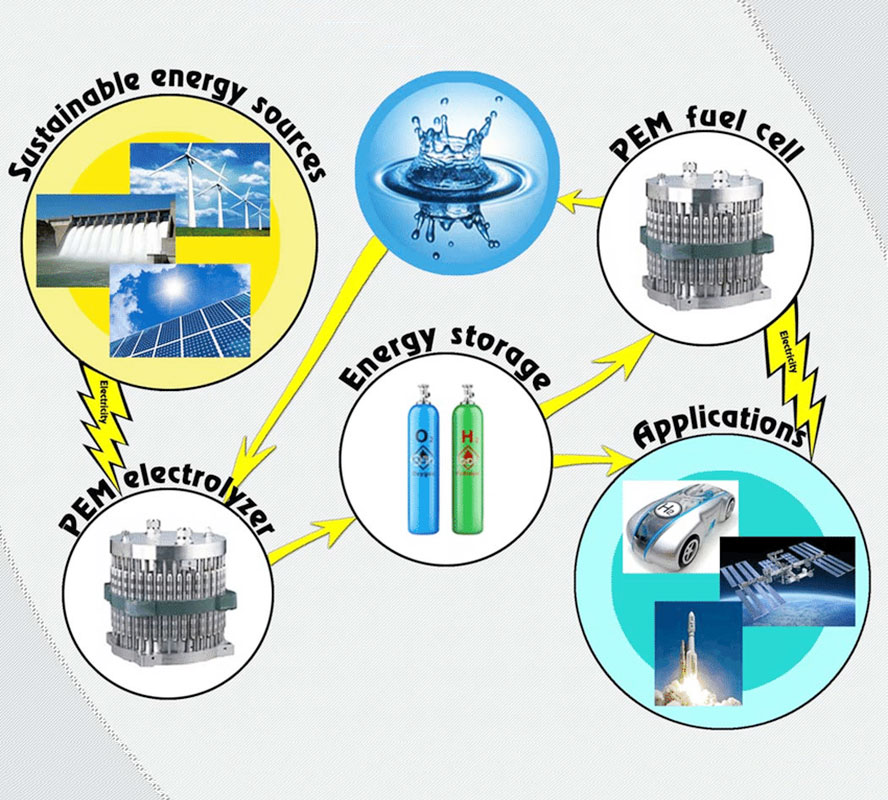
The significance of safe, functional, and efficient fuel cell systems, along with their critical companions in progress – electrolysers and hydrogen refuelling stations (HRS) – cannot be overstated in the journey towards sustainable energy. They form the bedrock of this transition, seamlessly merging the realms of innovation, reliability, and environmental responsibility.
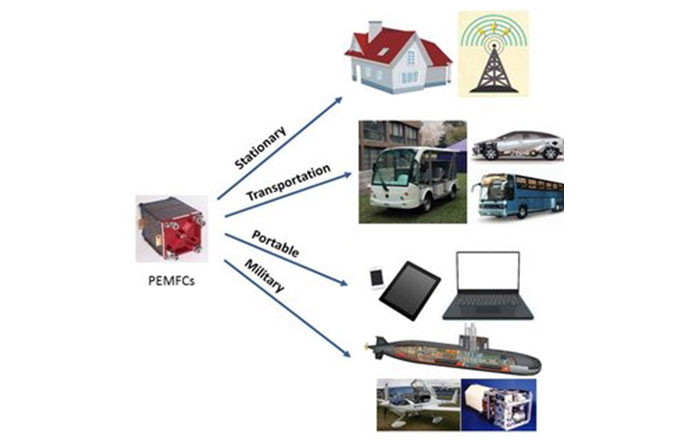
Today’s landscape of fuel cell systems spans the gamut, ranging from the compact, yet potent, portable applications generating a few Watts to the colossal field installations boasting megawatt-scale power supply. This breath-taking diversity underscores the adaptability and versatility of fuel cells, underlining their potential to revolutionize energy generation across multiple scales, ultimately driving the global shift towards a more sustainable future.
Upon interacting with the catalyst, hydrogen undergoes a remarkable transformation, splitting into two essential components: protons & electrons. What follows is a dance of energy conversion orchestrated at the atomic level.
Protons, driven by an almost unerring sense of purpose, traverse a specialized membrane with remarkable ease, unhindered by obstacles, and head determinedly towards the cathode. Meanwhile, the electrons embark on an external circuit, compelled to embark on a journey that will power our world.
As these electrons navigate their predetermined path through the external circuit, they unravel their potential & give birth to electricity. This electricity, an embodiment of energy in motion, stands ready to serve myriad purposes, whether it’s the gentle illumination of a humble light bulb or the vigorous propulsion of a mighty motor.
Ultimately, hydrogen’s protons & electrons reunite in a spectacular finale, joining forces with oxygen in a grand chemical ballet. This captivating fusion culminates in the creation of water, nature’s own testament to the beauty of this electrochemical symphony.
Why Fuel Cells?

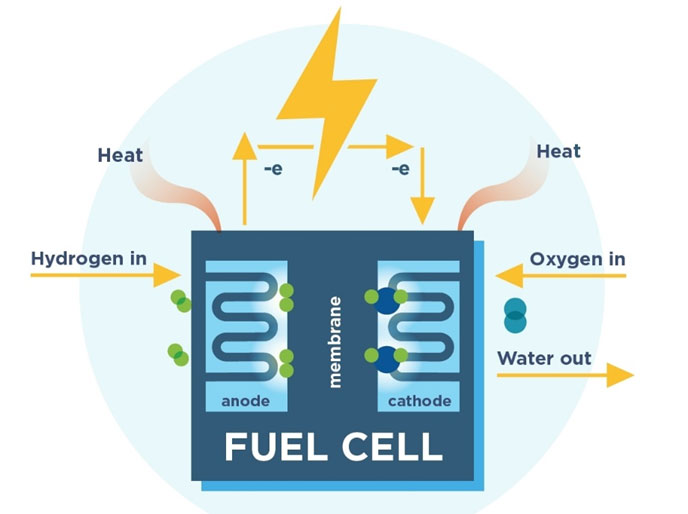
How Do Fuel Cells Work?
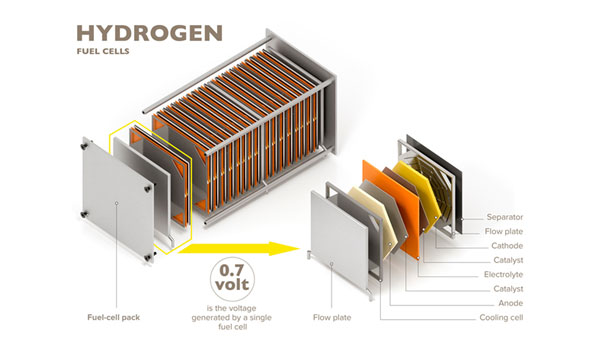
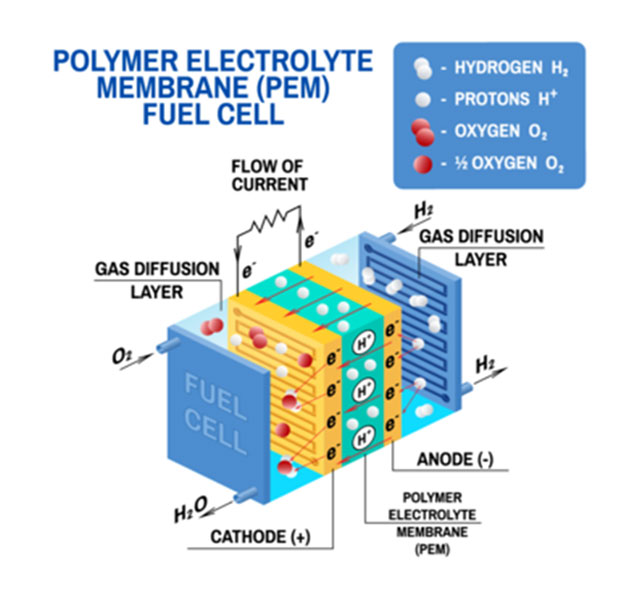
A single fuel cell consists of an electrolyte sandwiched between two electrodes, an anode and a cathode. Bipolar plates on either side of the cell help distribute gases and serve as current collectors. In a Polymer Electrolyte Membrane (PEM) fuel cell, which is widely regarded as the most promising for light-duty transportation, hydrogen gas flows through channels to the anode, where a catalyst causes the hydrogen molecules to separate into protons and electrons.
The membrane allows only the protons to pass through it. While the protons are conducted through it. While the protons are conducted through the membrane to the other side of the cell, the stream of negatively-charged electrons follows an external circuit to the cathode. This flow of electrons is electricity that can be used to do work, such as power a motor.
On the other side of the cell, oxygen gas, typically drawn from the outside air, flows through channels to the cathode. When the electrons return from doing work, they react with oxygen and the hydrogen protons (which have moved through the membrane) at the cathode to form water. This union is an exothermic reaction, generating heat that can be used outside the fuel cell.
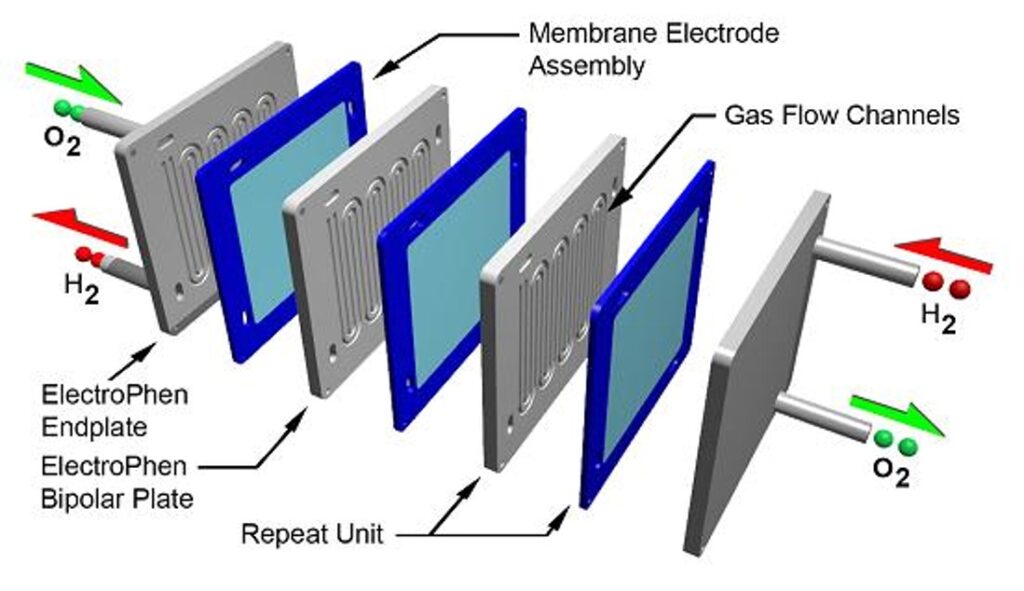
The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure at which gases are supplied. A single fuel cell produces approximately 1 volt or less — barely enough electricity for even the smallest applications. To increase the amount of electricity generated, individual fuel cells are combined in series to form a stack. (The term “fuel cell” is often used to refer to the entire stack, as well as to the individual cell.) Depending on the application, a fuel cell stack may contain only a few or as many as hundreds of individual cells layered together. This “Scalability” makes fuel cells ideal for a wide variety of applications, from laptop computers (50-100 Watts) to homes (1-5kW), vehicles (50-125 kW), and central power generation (1-200 MW or more).
By converting potential chemical energy directly into electrical energy, fuel cells avoid a thermal bottleneck (a consequence of the second law of thermodynamics) & are therefore inherently more efficient than combustion engines, which must first convert potential chemical energy into heat, then into mechanical work.
Pure water comes out of the system’s exhaust pipe, with no emissions of particulate matter or other gases, with a significant environmental advantage over an internal combustion engine.
Fuel cells have few moving parts, which increases reliability and reduces maintenance, compared to an internal combustion engine.
When hydrogen is generated from renewable electricity sources, such as wind, solar or hydropower, the related CO2 emissions are extremely low.
The ability of hydrogen to combine with oxygen was first noted by Henry Cavendish in 1766. The first electrolyze appeared later in 1800, when Nicholson and Carlisle induced a static charge in water.

In alkaline electrolysis, a reaction occurs between two electrodes in a solution composed of water and a liquid electrolyte. When sufficient voltage is applied, water molecules take electrons to produce OH- ions and an H2 molecule. OH- ions travel through the solution to an anode, where they combine and lose their additional electrons to produce water, electrons and O2.
The recombination of hydrogen and oxygen, in this phase, is avoided by means of special ion exchange membranes. The electrolyte remains in the system due to a closed-loop, pumpless recirculation process.
PEM electrolysis creates a reaction using an ionically conductive solid polymer, instead of a liquid. When voltage is applied between the two electrodes, negatively charged oxygen in water molecules releases its electron, which results in protons, electrons and O2 at the anode. H+ ions travel through the proton-conducting polymer to the cathode, where they take an electron and become neutral H atoms. The latter combine to produce H2 at the cathode.
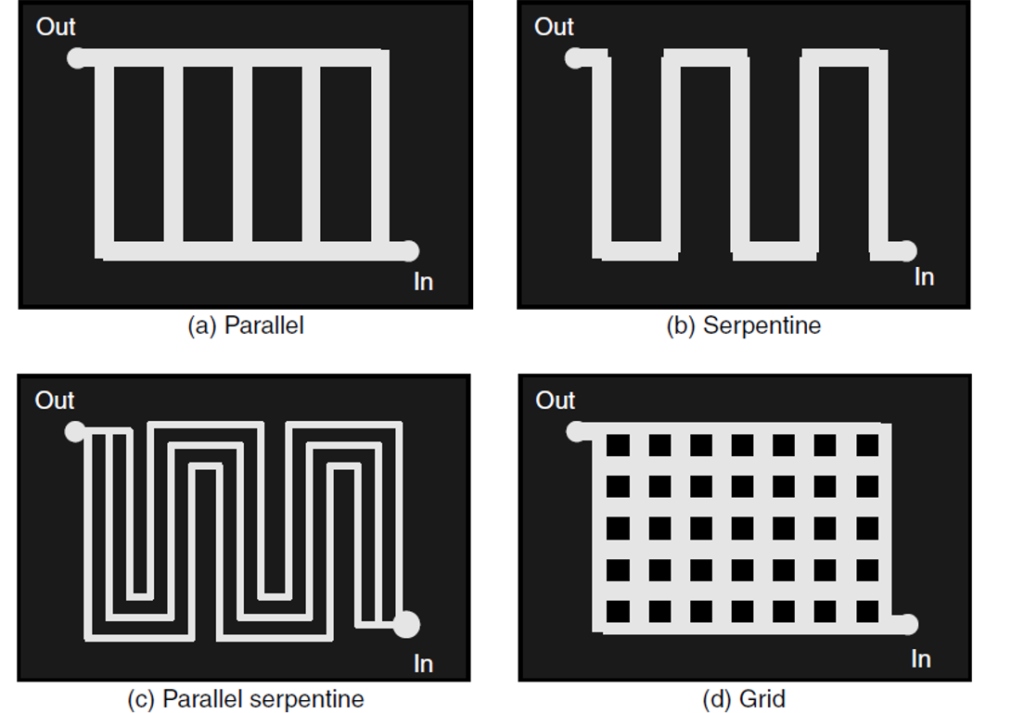
The electrolyte and the two electrodes are enclosed between two bipolar plates, which transport water to them, transport gaseous products away from the cell, conduct electricity and circulate a refrigerant fluid to cool the process.